Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Shinohara, Yuya*; Iwashita, Takuya*; Nakanishi, Masahiro*; Osti, N. C.*; Kofu, Maiko; Nirei, Masami; Dmowski, W.*; Egami, Takeshi*
Journal of Physical Chemistry B, 128(6), p.1544 - 1549, 2024/02
Times Cited Count:0 Percentile:0.01(Chemistry, Physical)Okazaki, Hiroyuki*; Idesaki, Akira*; Koshikawa, Hiroshi*; Matsumura, Daiju; Ikeda, Takashi*; Yamamoto, Shunya*; Yamaki, Tetsuya*
Journal of Physical Chemistry C, 127(49), p.23628 - 23633, 2023/12
Times Cited Count:0 Percentile:0(Chemistry, Physical)Tsuji, Hayato*; Nakahata, Masaki*; Hishida, Mafumi*; Seto, Hideki*; Motokawa, Ryuhei; Inoue, Takeru*; Egawa, Yasunobu*
Journal of Physical Chemistry Letters (Internet), 14(49), p.11235 - 11241, 2023/12
Times Cited Count:0 Percentile:0.01(Chemistry, Physical)Tamura, Kazuhisa
Journal of Physical Chemistry C, 127(46), p.22733 - 22739, 2023/11
Times Cited Count:0 Percentile:0(Chemistry, Physical)The underpotential deposition of Bi on Au(111) electrode in 1 M HClO solution and 1-butyl-3-methylimidazolium tetrafluoroborate was investigated using visible light reflectance measurement and surface X-ray scattering techniques. The electrosorption valency of the UPD reaction of Bi was elucidated and it was found that in both 1 M HClO
solution and 1-butyl-3-methylimidazolium tetrafluoroborate was investigated using visible light reflectance measurement and surface X-ray scattering techniques. The electrosorption valency of the UPD reaction of Bi was elucidated and it was found that in both 1 M HClO and 1-butyl-3-methylimidazolium tetrafluoroborate the electrosorption valency was smaller than 3, but the detail process in the UPD reaction was different between in two electrolytes. The difference may be originated from the difference in the solvation status of Bi
and 1-butyl-3-methylimidazolium tetrafluoroborate the electrosorption valency was smaller than 3, but the detail process in the UPD reaction was different between in two electrolytes. The difference may be originated from the difference in the solvation status of Bi rather than the electrical double layer structure.
rather than the electrical double layer structure.
 neutron diffraction
neutron diffractionKobayashi, Hiroki*; Komatsu, Kazuki*; Ito, Hayate*; Machida, Shinichi*; Hattori, Takanori; Kagi, Hiroyuki*
Journal of Physical Chemistry Letters (Internet), 14(47), p.10664 - 10669, 2023/11
Times Cited Count:0 Percentile:0.01(Chemistry, Physical)Ice IV is a metastable high-pressure phase of ice in which the water molecules exhibit orientational disorder. Although orientational ordering is commonly observed for other ice phases, it has not been reported for ice IV. We conducted  powder neutron diffraction experiments for DCl-doped D
powder neutron diffraction experiments for DCl-doped D O ice IV to investigate hydrogen ordering in ice IV. We found abrupt changes in the temperature derivative of unit cell volume, dV/dT, at about 120 K, and revealed their slightly ordered structure at low temperatures based on the Rietveld method. The occupancy of the D1 site deviates from 0.5; it increased when samples were cooled at higher pressures and reached 0.282(5) at 2.38 GPa, 58 K. Our results evidence the presence of a low-symmetry hydrogen-ordered state corresponding to ice IV. It seems, however, difficult to experimentally access the completely ordered phase corresponding to ice IV by slow cooling at high pressure.
O ice IV to investigate hydrogen ordering in ice IV. We found abrupt changes in the temperature derivative of unit cell volume, dV/dT, at about 120 K, and revealed their slightly ordered structure at low temperatures based on the Rietveld method. The occupancy of the D1 site deviates from 0.5; it increased when samples were cooled at higher pressures and reached 0.282(5) at 2.38 GPa, 58 K. Our results evidence the presence of a low-symmetry hydrogen-ordered state corresponding to ice IV. It seems, however, difficult to experimentally access the completely ordered phase corresponding to ice IV by slow cooling at high pressure.
Kumada, Takayuki; Nakagawa, Hiroshi; Miura, Daisuke; Sekine, Yurina; Motokawa, Ryuhei; Hiroi, Kosuke; Inamura, Yasuhiro; Oku, Takayuki; Oishi, Kazuki*; Morikawa, Toshiaki*; et al.
Journal of Physical Chemistry Letters (Internet), 14(34), p.7638 - 7643, 2023/08
Times Cited Count:0 Percentile:0.01(Chemistry, Physical)The structure of nano-ice crystals in rapidly frozen glucose solution was elucidated by using spin-contrast-variation small-angle neutron scattering, which distinguishes the nano-ice crystal signal from the frozen amorphous solution signal by the polarization-dependent neutron scattering. The analysis revealed that the nano-ice crystals form a planar structure with a diameter exceeding tens of nanometers and a thickness of 1 nm, which is close to the critical nucleation size. This result suggests that the glucose molecules are preferentially bound to a specific face of nano-ice crystals, and then block the crystal growth perpendicular to that face.
 ortho-para conversion on a metal surface; Interplay between electron and phonon systems
ortho-para conversion on a metal surface; Interplay between electron and phonon systemsUeta, Hirokazu; Fukutani, Katsuyuki
Journal of Physical Chemistry Letters (Internet), 14(34), p.7591 - 7596, 2023/08
Inagawa, Kohei*; Matsumura, Daiju; Taniguchi, Masashi*; Uegaki, Shinya*; Nakayama, Tomohito*; Urano, Junnosuke*; Aotani, Takuro*; Tanaka, Hirohisa*
Journal of Physical Chemistry C, 127(24), p.11542 - 11549, 2023/06
Times Cited Count:0 Percentile:0(Chemistry, Physical)Massey, D.*; Williams, C. D.*; Mu, J.*; Masters, A. J.*; Motokawa, Ryuhei; Aoyagi, Noboru; Ueda, Yuki; Antonio, M. R.*
Journal of Physical Chemistry B, 127(9), p.2052 - 2065, 2023/03
Times Cited Count:0 Percentile:0(Chemistry, Physical)Balois-Oguchi, M. V.*; Hayazawa, Norihiko*; Yasuda, Satoshi; Ikeda, Katsuyoshi*; Nguyen, T. Q.*; Esca o, M. C.*; Tanaka, Takuo*
o, M. C.*; Tanaka, Takuo*
Journal of Physical Chemistry C, 127(12), p.5982 - 5990, 2023/03
Times Cited Count:2 Percentile:52.07(Chemistry, Physical)Micrometer-sized wrinkles in graphene are known to affect the electronic properties of graphene due to their shape and the strain variations they create. Here, we analyze the strain distribution and doping of a graphene wrinkle having 1.9 nm width using tip-enhanced Raman spectroscopy (TERS) in ambient conditions. We found a strong correlation between the TERS images of the graphene wrinkle and the electronic Raman scattering (eRS) of the Au(111) substrate. Our work demonstrates that the as-fabricated physical and electronic properties of nanometer-sized features, such as wrinkles, can be probed and studied in detail with TERS which is essential for nanodevice characterization.
Ye, X.*; Shimokawa, Kohei*; Kezuka, Yuto*; Hatakeyama, Takuya*; Li, H.*; Ichitsubo, Tetsu
Journal of Physical Chemistry C, 127(11), p.5210 - 5218, 2023/03
Times Cited Count:1 Percentile:52.07(Chemistry, Physical) Cs NMR chemical shift of clay minerals via machine learning and DFT-GIPAW calculations
Cs NMR chemical shift of clay minerals via machine learning and DFT-GIPAW calculationsOkubo, Takahiro*; Takei, Akihiro*; Tachi, Yukio; Fukatsu, Yuta; Deguchi, Kenzo*; Oki, Shinobu*; Shimizu, Tadashi*
Journal of Physical Chemistry A, 127(4), p.973 - 986, 2023/02
Times Cited Count:1 Percentile:56.86(Chemistry, Physical)The identification of adsorption sites of Cs on clay minerals has been studied in the fields of environmental chemistry. The nuclear magnetic resonance (NMR) experiments allow direct observations of the local structures of adsorbed Cs. The NMR parameters of  Cs, derived from solid-state NMR experiments, are sensitive to the local neighboring structures of adsorbed Cs. However, determining the Cs positions from NMR data alone is difficult. This paper describes an approach for identifying the expected atomic positions of Cs adsorbed on clay minerals by combining machine learning (ML) with experimentally observed chemical shifts. A linear ridge regression model for ML is constructed from the smooth overlap of atomic positions descriptor and gauge-including projector augmented wave (GIPAW) ab initio data. The
Cs, derived from solid-state NMR experiments, are sensitive to the local neighboring structures of adsorbed Cs. However, determining the Cs positions from NMR data alone is difficult. This paper describes an approach for identifying the expected atomic positions of Cs adsorbed on clay minerals by combining machine learning (ML) with experimentally observed chemical shifts. A linear ridge regression model for ML is constructed from the smooth overlap of atomic positions descriptor and gauge-including projector augmented wave (GIPAW) ab initio data. The  Cs chemical shifts can be instantaneously calculated from the Cs positions on any clay layers using ML. The inverse analysis from the ML model can derive the atomic positions from experimentally observed chemical shifts.
Cs chemical shifts can be instantaneously calculated from the Cs positions on any clay layers using ML. The inverse analysis from the ML model can derive the atomic positions from experimentally observed chemical shifts.
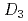 point group symmetry (M(II) = Zn(II) and Mg(II))
point group symmetry (M(II) = Zn(II) and Mg(II))Masuda, Yuka*; Sakata, Shiomi*; Kayahara, Saori*; Irie, Natsumi*; Kofu, Maiko; Kono, Yohei*; Sakakibara, Toshiro*; Horii, Yoji*; Kajiwara, Takashi*
Journal of Physical Chemistry C, 127(6), p.3295 - 3306, 2023/02
Times Cited Count:1 Percentile:52.07(Chemistry, Physical) NH and CaNH thin films by reactive magnetron sputtering under hydrogen partial pressure control
NH and CaNH thin films by reactive magnetron sputtering under hydrogen partial pressure controlChon, S.*; Fukutani, Katsuyuki; 8 of others*
Journal of Physical Chemistry Letters (Internet), 13(43), p.10169 - 10174, 2022/11
Times Cited Count:0 Percentile:0.01(Chemistry, Physical)Kusaka, Ryoji; Watanabe, Masayuki
Journal of Physical Chemistry Letters (Internet), 13(30), p.7065 - 7071, 2022/08
Times Cited Count:5 Percentile:70.33(Chemistry, Physical) GeP
GeP S
S by quasi-elastic neutron scattering measurements
by quasi-elastic neutron scattering measurementsHori, Satoshi*; Kanno, Ryoji*; Kwon, O.*; Kato, Yuki*; Yamada, Takeshi*; Matsuura, Masato*; Yonemura, Masao*; Kamiyama, Takashi*; Shibata, Kaoru; Kawakita, Yukinobu
Journal of Physical Chemistry C, 126(22), p.9518 - 9527, 2022/06
Times Cited Count:3 Percentile:41.53(Chemistry, Physical) ion complex; Presence of a Rh
ion complex; Presence of a Rh Intermediate in Direct Photoreduction
Intermediate in Direct PhotoreductionSaeki, Morihisa*; Matsumura, Daiju; Nakanishi, Ryuzo*; Yomogida, Takumi; Tsuji, Takuya; Saito, Hiroyuki*; Oba, Hironori*
Journal of Physical Chemistry C, 126(12), p.5607 - 5616, 2022/03
Times Cited Count:1 Percentile:14.66(Chemistry, Physical)The reaction mechanism of the direct photoreduction of a Rh ion complex to a Rh
ion complex to a Rh species induced by pulsed ultraviolet laser irradiation was studied using dispersive X-ray absorption fine structure (DXAFS) spectroscopy. The time-resolved X-ray absorption near edge structure (XANES) showed the absence of isosbestic points and suggested that more than two Rh
species induced by pulsed ultraviolet laser irradiation was studied using dispersive X-ray absorption fine structure (DXAFS) spectroscopy. The time-resolved X-ray absorption near edge structure (XANES) showed the absence of isosbestic points and suggested that more than two Rh species contribute toward the direct photoreduction of Rh
species contribute toward the direct photoreduction of Rh . Analysis of the time-resolved XANES data by singular value deposition showed that the direct photoreduction involves three Rh
. Analysis of the time-resolved XANES data by singular value deposition showed that the direct photoreduction involves three Rh species. Multivariate curve resolution by alternating least-squares analysis (MCR-ALS) of the time-resolved XANES data gave pure spectra and concentration profiles of the three Rh
species. Multivariate curve resolution by alternating least-squares analysis (MCR-ALS) of the time-resolved XANES data gave pure spectra and concentration profiles of the three Rh species. The Rh
species. The Rh species were assigned to Rh
species were assigned to Rh , Rh
, Rh , and Rh
, and Rh species based on the features of the pure XANES spectra. The concentration profiles suggested that the direct photoreduction proceeds in the order of Rh
species based on the features of the pure XANES spectra. The concentration profiles suggested that the direct photoreduction proceeds in the order of Rh
 Rh
Rh
 Rh
Rh . A reaction mechanism, which was proposed involving photoreductions of Rh
. A reaction mechanism, which was proposed involving photoreductions of Rh and Rh
and Rh , photoinduced autocatalytic reductions of Rh
, photoinduced autocatalytic reductions of Rh and Rh
and Rh , and photooxidation of Rh
, and photooxidation of Rh , well reproduced the concentration profiles of three Rh
, well reproduced the concentration profiles of three Rh species.
species.
Wang, X.*; Tang, X.*; Zhang, P.*; Wang, Y.*; Gao, D.*; Liu, J.*; Hui, K.*; Wang, Y.*; Dong, X.*; Hattori, Takanori; et al.
Journal of Physical Chemistry Letters (Internet), 12(50), p.12055 - 12061, 2021/12
Times Cited Count:6 Percentile:44.89(Chemistry, Physical)Substituted polyacetylene is expected to improve the chemical stability, physical properties, and additional functions of the polyacetylene backbones, but its diversity is very limited. Here, by applying external pressure on solid acetylenedicarboxylic acid, we report the first crystalline poly-dicarboxylacetylene with every carbon on the trans-polyacetylene backbone bonded to a carboxyl group, which is very hard to synthesize by traditional methods. This unique structure combines the extremely high content of carbonyl groups and high conductivity of a polyacetylene backbone, which exhibits a high specific capacity and excellent cycling/rate performance as a Li-ion battery (LIB) anode. We present a completely functionalized crystalline polyacetylene and provide a high-pressure solution for the synthesis of polymeric LIB materials and other polymeric materials with a high content of active groups.
Ito, Kanae; Yamada, Takeshi*; Shinohara, Akihiro*; Takata, Shinichi; Kawakita, Yukinobu
Journal of Physical Chemistry C, 125(39), p.21645 - 21652, 2021/10
Times Cited Count:4 Percentile:29.65(Chemistry, Physical)Yasuda, Satoshi; Tamura, Kazuhisa; Kato, Masaru*; Asaoka, Hidehito; Yagi, Ichizo*
Journal of Physical Chemistry C, 125(40), p.22154 - 22162, 2021/10
Times Cited Count:10 Percentile:57.69(Chemistry, Physical)Understanding electrochemical behavior of the alkaline metal cation-graphene interface in electrolyte is essential for understanding the fundamental electrochemical interface and development of graphene-based technologies. We report comprehensive analysis of the electrochemical behavior of both alkaline metal cations and graphene using electrochemical surface X-ray diffraction (EC-SXRD) and Raman (EC-Raman) spectroscopic techniques in which the interfacial structure of cations and the charging state and mechanical strain of the graphene can be elucidated. EC-SXRD and cyclic voltammetry demonstrated electrochemically driven specific adsorption and desorption of cations on the graphene surface involved in the dehydration and hydration process. This study provides new insight for understanding fundamental electrochemical behavior of the alkaline metal cation-graphene interface and contributes to the development of carbon-based novel applications.